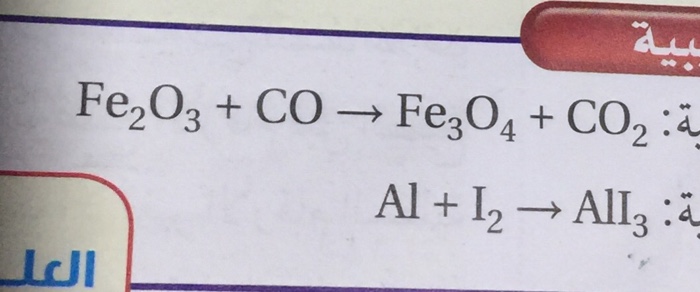Nanomaterials | Free Full-Text | Superparamagnetic Fe3O4@CA Nanoparticles and Their Potential as Draw Solution Agents in Forward Osmosis
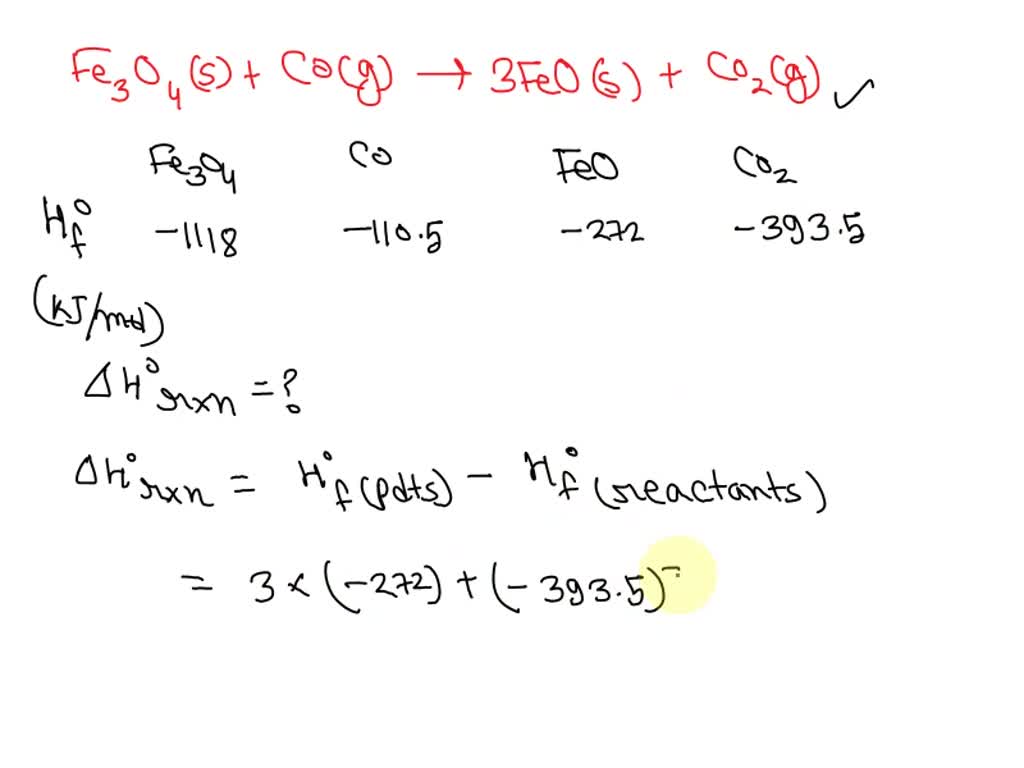
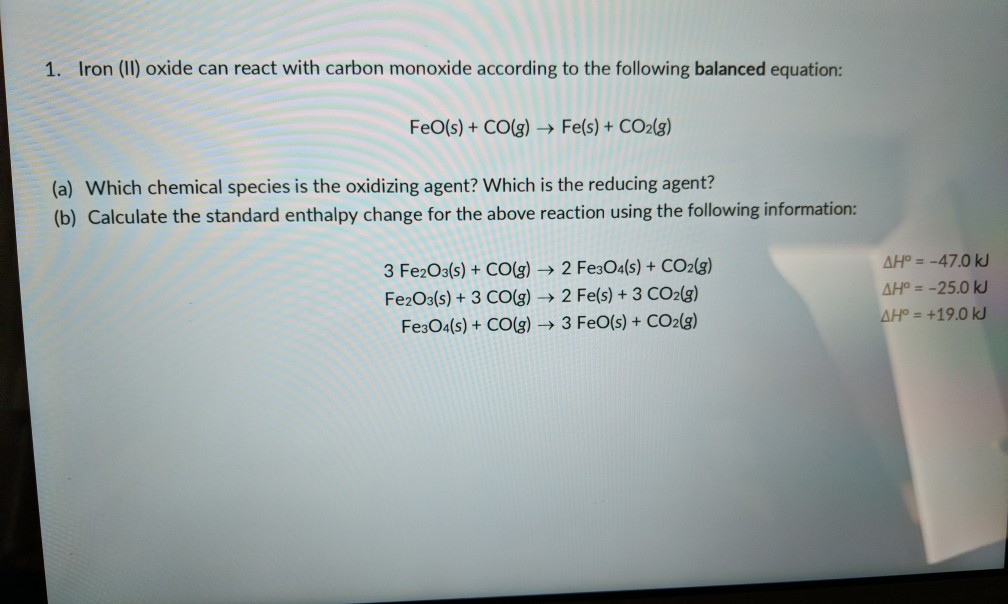
SOLVED: Calculate Horxn for the following reaction at 25.0oC: Fe3O4(s) + CO(g) –> 3FeO(s) + CO2(g) Hfo (kJ/mol) => Fe3O4(s) = -1118, CO(g) = -110.5, FeO(s) = -272, CO2(g) = -393.5

High Conversion to Aromatics via CO2-FT over a CO-Reduced Cu-Fe2O3 Catalyst Integrated with HZSM-5 | ACS Catalysis

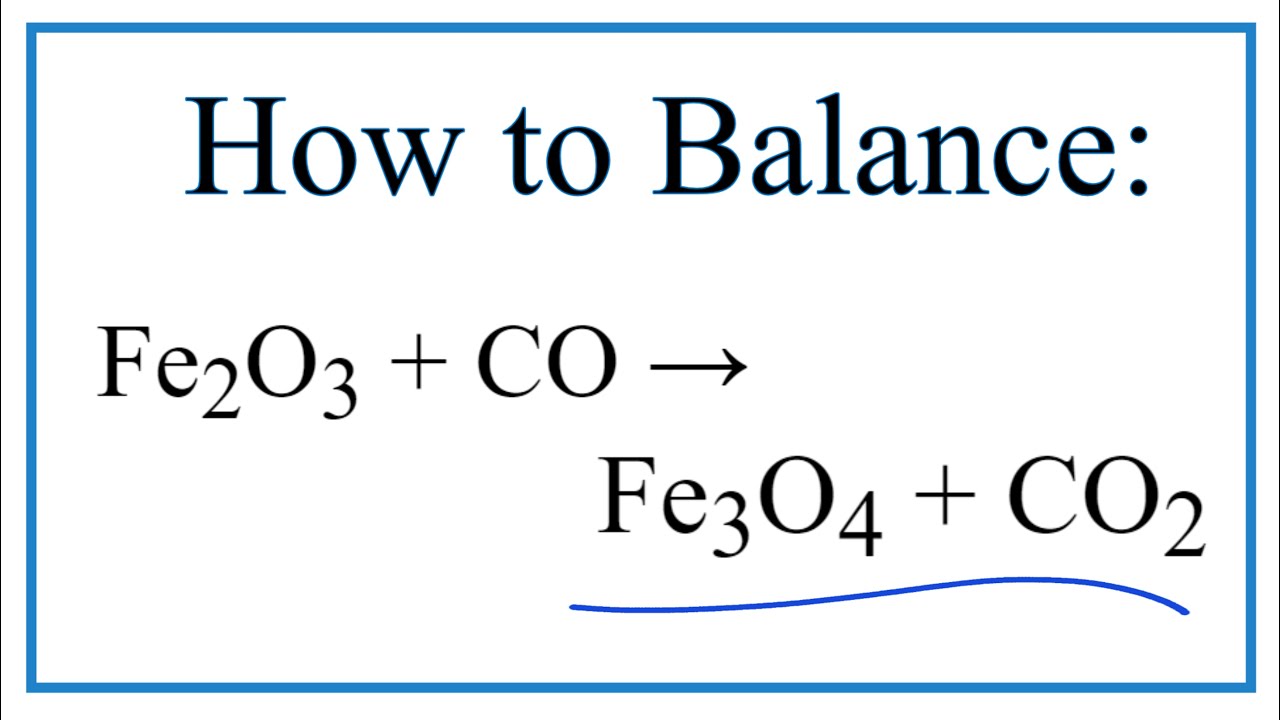
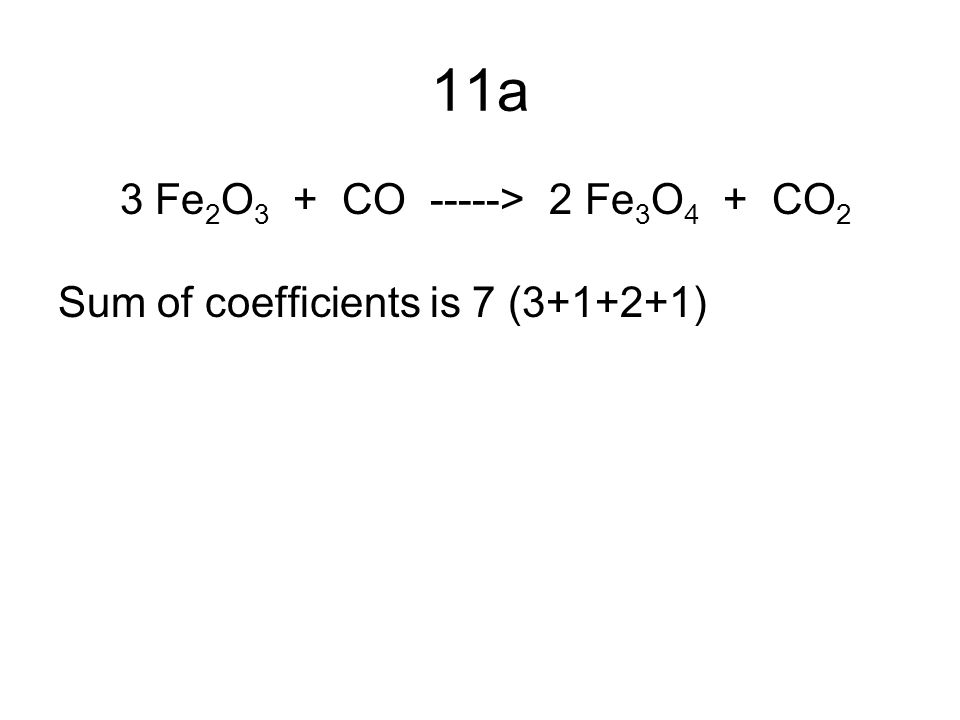
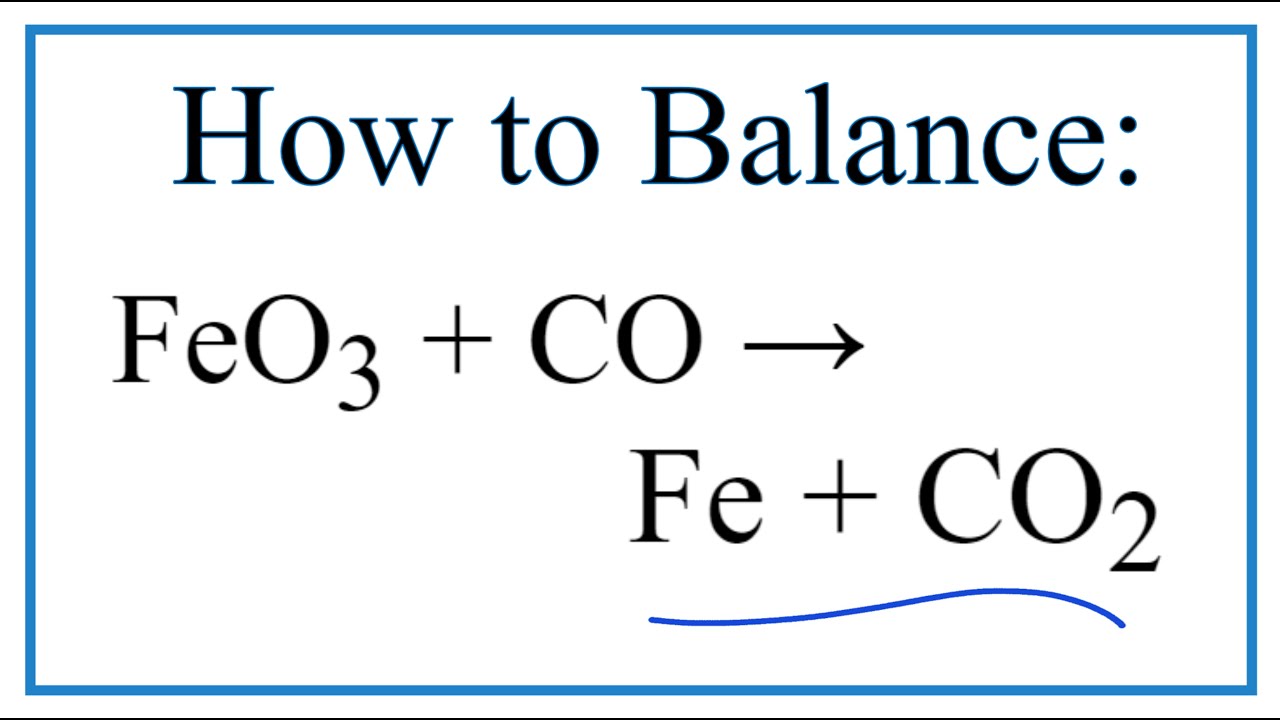
SOLVED: Question 29 (2 points) Which of these choices shows the following chemical reaction properly balanced? FezO3 + CO Fe3O4 CO2 FezO3 2CO Fe3O4 2C02 3FezO3 + CO 2Fe3O4 + CO2 None

The effect of Fe3O4 nanoparticles on the mass transfer of CO2 absorption into aqueous ammonia solutions - ScienceDirect

Life Cycle Impact Assessment of Iron Oxide (Fe3O4/γ-Fe2O3) Nanoparticle Synthesis Routes | ACS Sustainable Chemistry & Engineering

Effect of Rb promoter on Fe3O4 microsphere catalyst for CO2 hydrogenation to light olefins - ScienceDirect

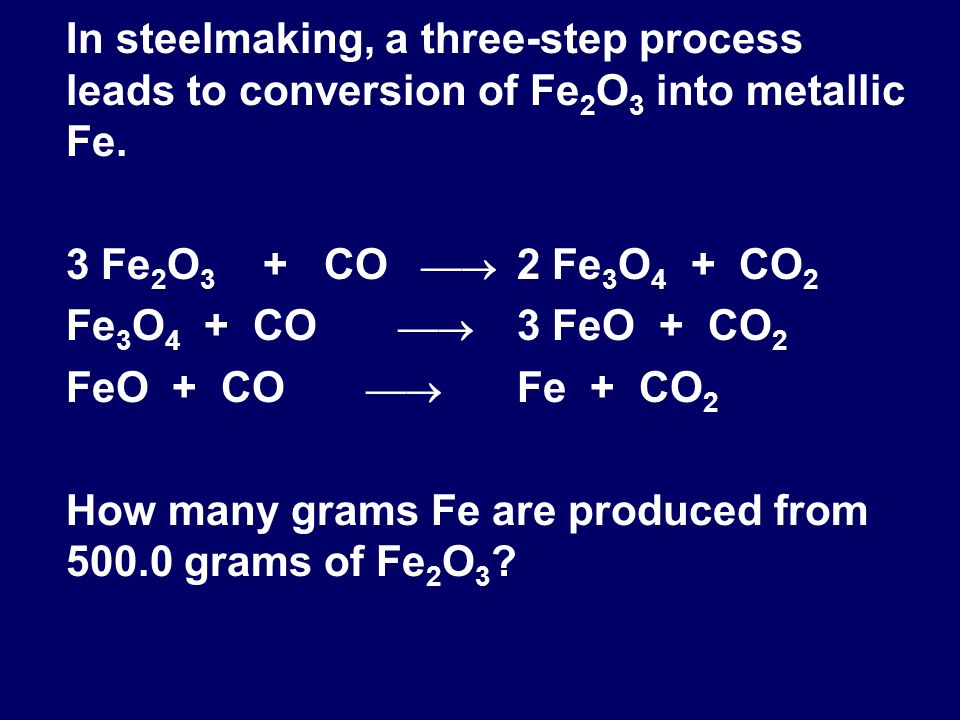
Which of the following reactions takes place at higher temperature range ( 900 K - 1500K) in blast furnace?
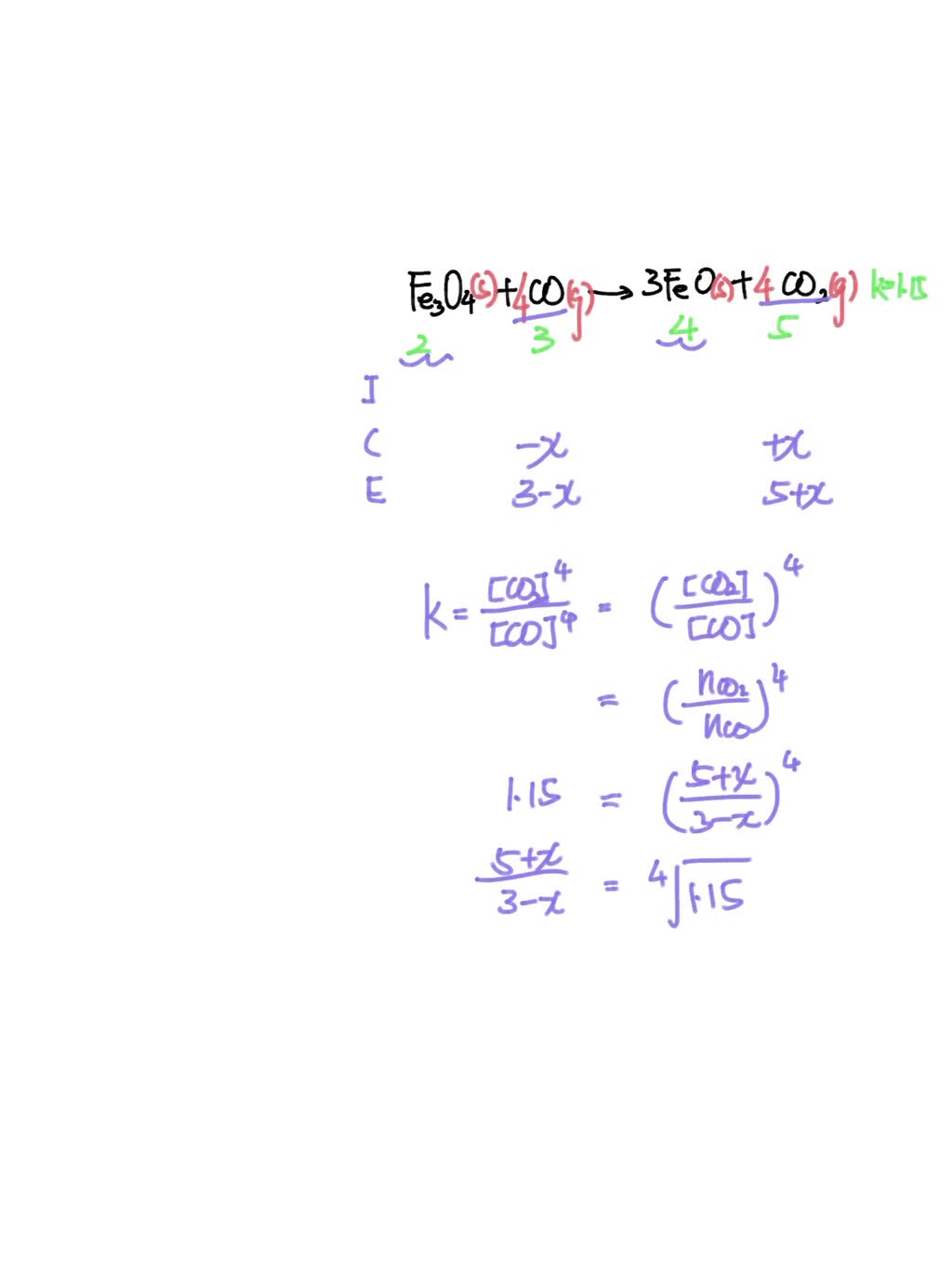
SOLVED: The equilibrium constant for the reaction Fe3O4 (s) + CO (g) ↔ 3FeO (s) + CO2 (g) It is 1.15 at 600 ° C. If a mixture of 2.00 moles of

An Atomic Insight into the Confusion on the Activity of Fe3O4 Nanoparticles as Peroxidase Mimetics and Their Comparison with Horseradish Peroxidase | The Journal of Physical Chemistry Letters