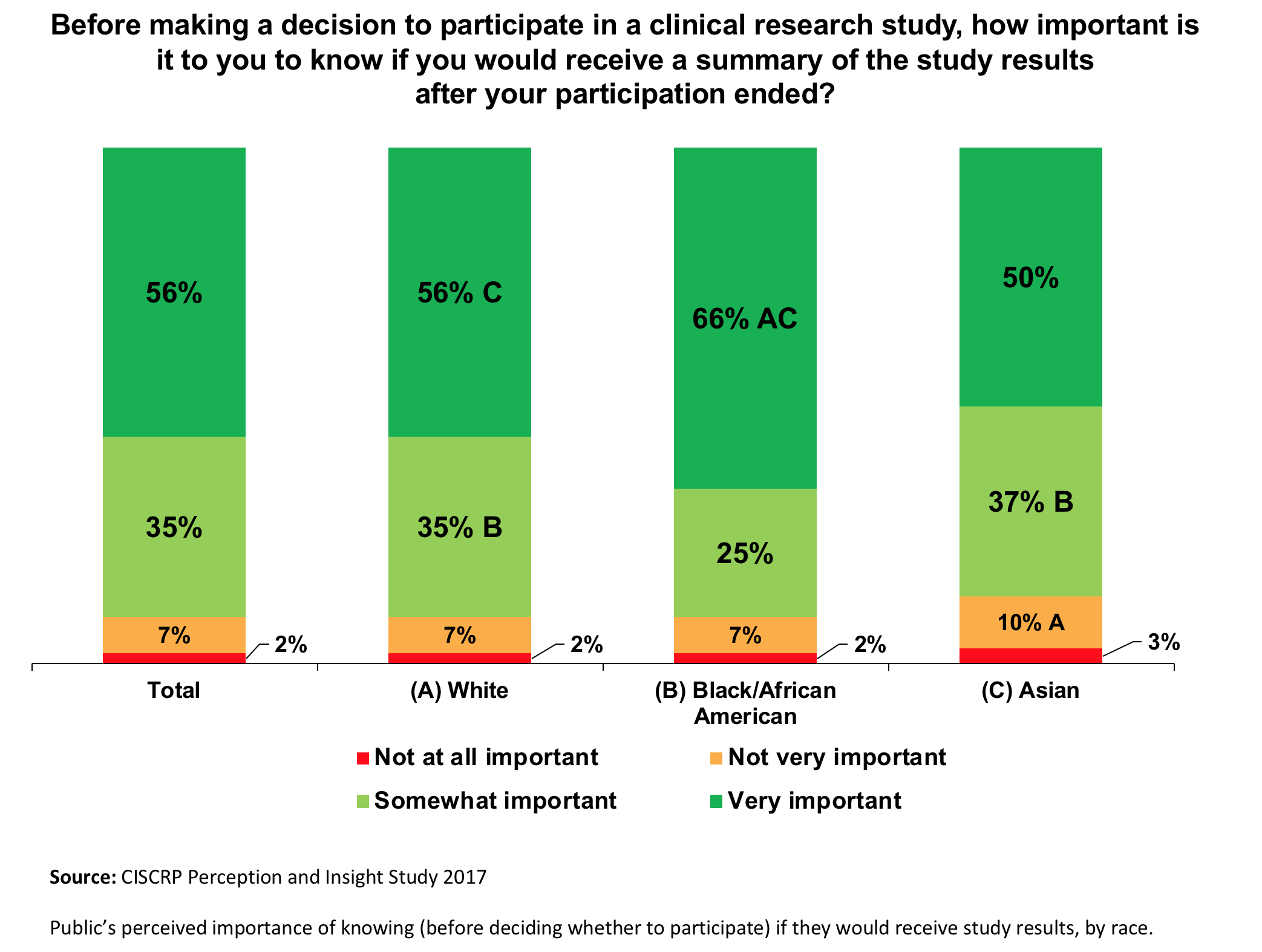
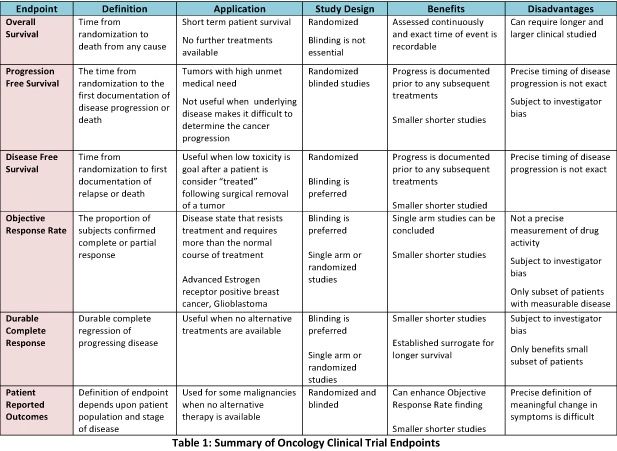
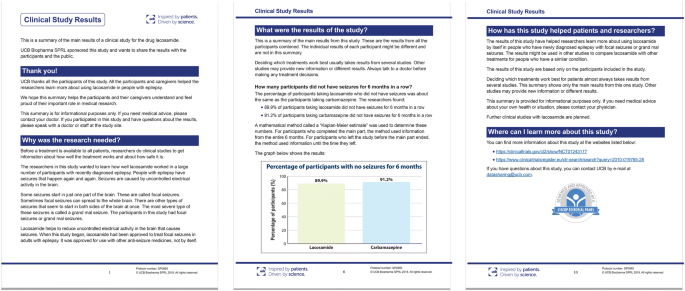
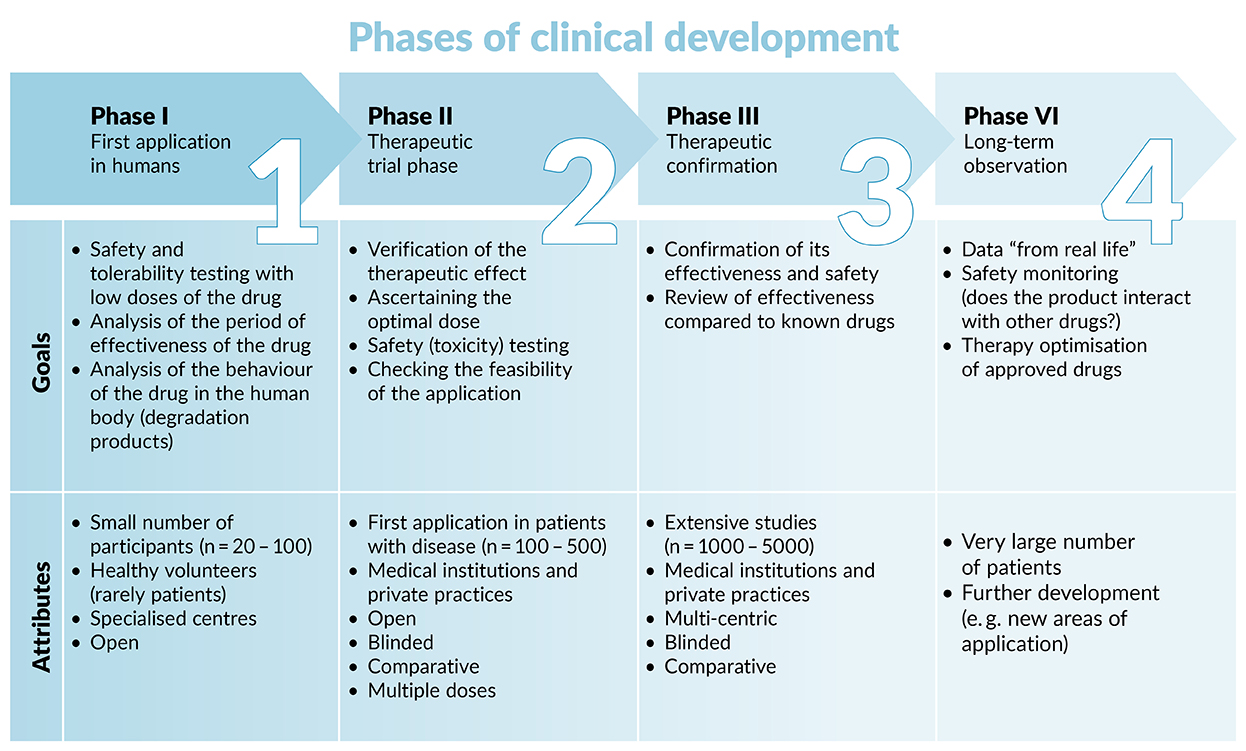
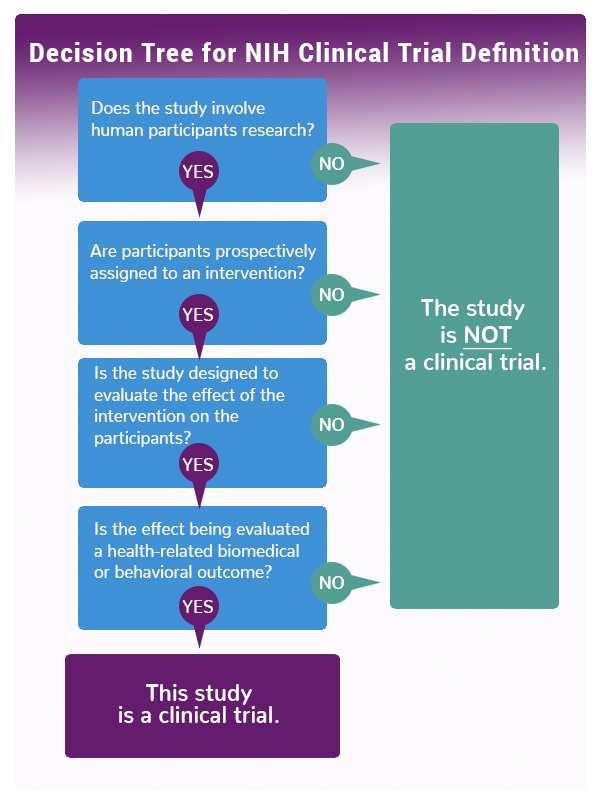
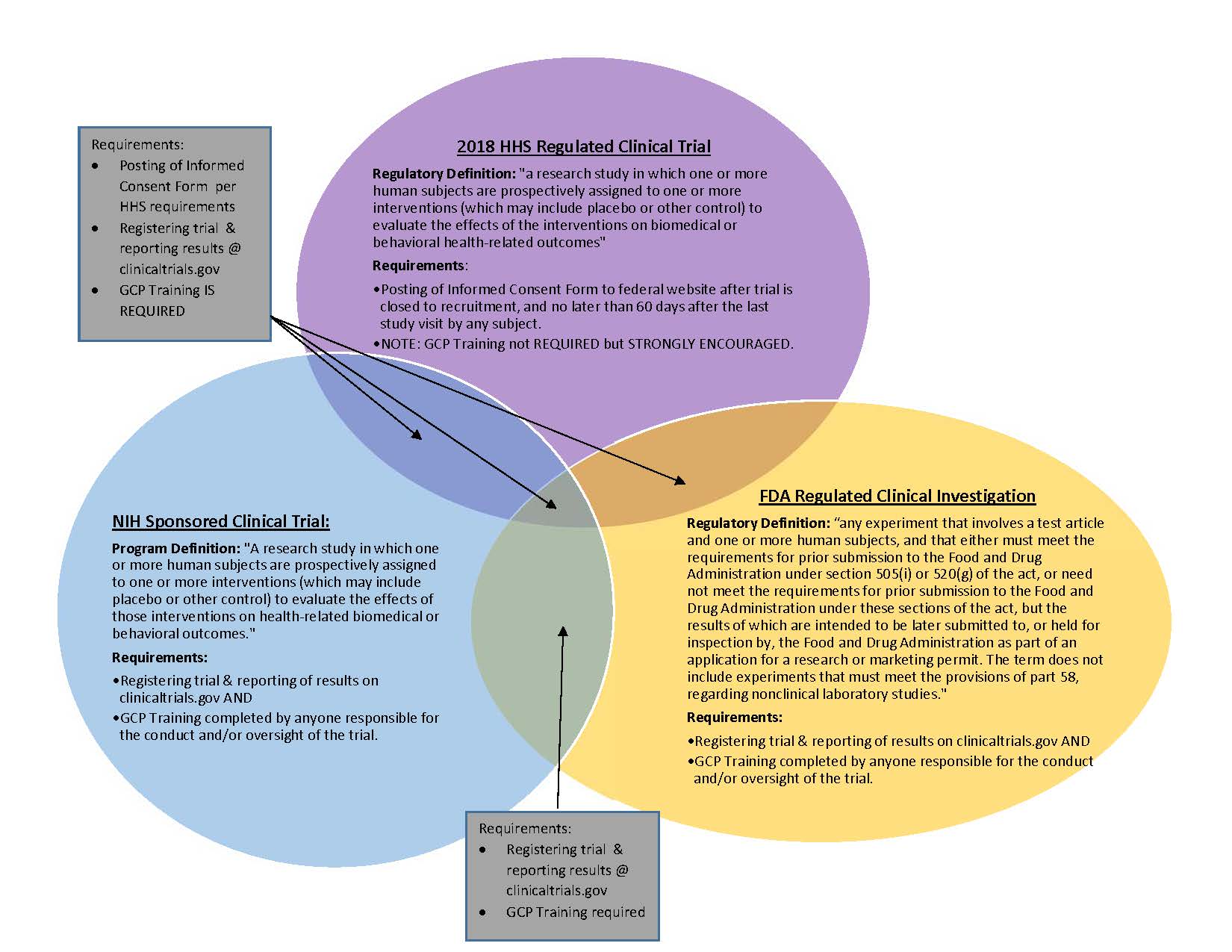
Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014-16: cross sectional analysis | The BMJ

Ongoing Clinical Trials for the Management of the COVID-19 Pandemic: Trends in Pharmacological Sciences
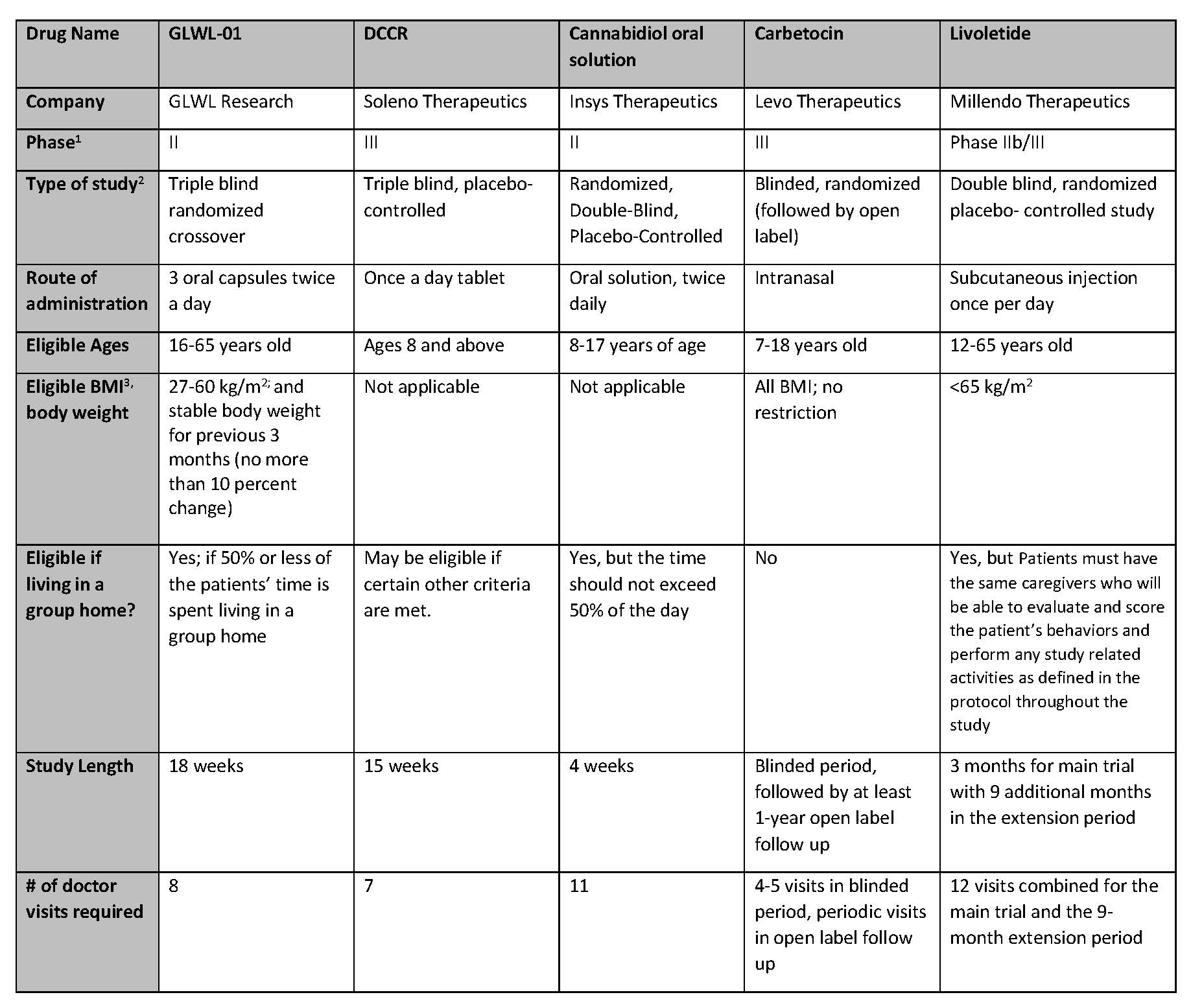
Summary Of Active Clinical Trials For Prader-Willi Syndrome Hyperphagia - Prader-Willi Syndrome Association | USA

Recruitment of Black Adults into Cardiovascular Disease Trials | Journal of the American Heart Association

Points in the clinical trial process (illustrated as dashed lines) at... | Download Scientific Diagram